How to Easy Your Sympotoms of Post Concussion Syndrom
Most young people who sustain a concussion during active play or sports naturally progress from the injury event through a period of symptom resolution, followed by a return to full normal activities. As discussed in Chapter 3, in 80 to 90 percent of cases, individuals' symptoms resolve within 2 weeks (Makdissi et al., 2010; McClincy et al., 2006; McCrea et al., 2009, 2013; McCrory et al., 2013), although recovery within that period appears to be somewhat slower for adolescents ages 13 years through high school than for college-age athletes (Covassin et al., 2010; Eisenberg et al., 2013; Field et al., 2003; McClincy et al., 2006). In 10 to 20 percent of individuals, however, concussive symptoms persist for a number of weeks, months, or even years. These individuals may be said to be experiencing post-concussion syndrome (PCS). This chapter reviews the diagnostic definitions of PCS, potential early predictors of prolonged recovery, and the symptomatology and management of individuals with prolonged recovery or PCS. The chapter addresses those areas described in the statement of task that pertain to risk factors for PCS; the cognitive, affective, and behavioral changes that may occur during the subacute and chronic posttraumatic phases; and the treatment and management of PCS. The committee's charge did not include the development or recommendation of specific clinical practice guidelines. Given that, the chapter is limited to a review and discussion of the available literature on the treatment and management of individuals experiencing prolonged recovery from a concussion.1
DIAGNOSTIC DEFINITION
Post-concussion syndrome is the persistence of a constellation of physical, cognitive, emotional, and sleep symptoms beyond the usual recovery period after a concussion. The World Health Organization's International Classification of Diseases, 10th revision (ICD-10), defines PCS2 as
a syndrome that occurs following head trauma (usually sufficiently severe to result in loss of consciousness) and includes a number of disparate symptoms such as headache, dizziness, fatigue, irritability, difficulty in concentration and performing mental tasks, impairment of memory, insomnia, and reduced tolerance to stress, emotional excitement, or alcohol. (WHO, 2010, F07.2)
The Diagnostic and Statistical Manual of Mental Disorders (DSM) no longer contains a specific entry for "postconcussional disorder." Instead, the fifth edition of the DSM captures persistent concussive symptoms under "neurocognitive disorder due to traumatic brain injury" (APA, 2013, pp. 624ff). The diagnostic criteria specify that the neurocognitive disorder must persist "past the acute post-injury period." There is concern that the symptoms associated with the diagnostic criteria for PCS are nonspecific and that they frequently occur in the absence of head injury and in conjunction with other psychiatric conditions (Ruff, 2011).
SHORT-TERM PREDICTORS OF A PROLONGED SYMPTOMATIC PERIOD POST CONCUSSION
Several studies have looked at different approaches to predicting which athletes will be most likely to have a prolonged recovery (typically more than 2 weeks post injury). A study of high school and college athletes with concussions found that loss of consciousness, posttraumatic and retrograde amnesia, and greater symptom severity (an increase of 20 points or more on the Graded Symptom Checklist) within the first 24 hours following injury were associated with longer recoveries (7 or more days) (McCrea et al., 2013). The researchers also found that demographic variables, competition level (high school versus college), player position, mechanism of injury, concussion history, and acute scores on the Standardized Assessment of Concussion (SAC) and the Balance Error Scoring System (BESS) tests were not predictive of a longer recovery time (McCrea et al., 2013).
Chrisman and colleagues (2013) used High School RIO™ data to identify predictors of prolonged symptoms, defined as lasting 7 or more days, in high school athletes. Presenting with four or more symptoms of concussion doubled the risk of prolonged symptoms. A history of prior concussion doubled the risk for prolonged symptoms in football players only. Symptoms of drowsiness, nausea, and concentration problems were associated with prolonged symptoms in all athletes, while sensitivity to light and noise was associated with prolonged symptoms only in non-football players. Amnesia was a risk factor only in males, and, contrary to the findings of McCrea and colleagues (2013), loss of consciousness was not a predictor of prolonged symptoms.
Iverson (2007) studied 114 concussed high school football players and found that slower-to-recover concussed athletes (those taking more than 10 days to recover) were more likely to have low scores on three neuropsychological tests (visual memory, reaction time, and processing speed). Lau and colleagues (2011b) examined 107 male high school football athletes who experienced a concussion and were divided into those with rapid (defined as ≤7 days) or prolonged (≥21 days) recovery. On-field dizziness was associated with a 6.3-fold increase in the odds of a prolonged recovery.
Related reports on the same sample that compared those with short (≤14 days) and prolonged (>14 days) recovery found that abnormal scores on both Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) neurocognitive testing and the Post-Concussion Symptom Scale (PCSS) at 2 days after injury could identify those who would have a prolonged recovery (Lau et al., 2011a, 2012). In an attempt to quantify the predictive value of symptoms and test scores for identifying protracted recovery, Lau and colleagues (2011a) followed 108 male high school football players from the time of their first post-injury evaluation (median of 2 days) until they returned to play. The athletes were classified as having a protracted recovery (>14 days; n=50) or a short recovery (≤14 days; n=58). Symptom clusters and neurocognitive composite scores used together had the highest sensitivity (65.22 percent), specificity (80.36 percent), positive predictive value (73.17 percent), and negative predictive value (73.80 percent) in predicting protracted recovery. Neither the symptom clusters nor the cognitive test scores alone provided adequate discriminating power. There was a net 24.41 increase in sensitivity when using neurocognitive testing and symptom clusters together compared with using total symptoms on PCSS alone.
A study of concussion in Australia elite football players found that delayed return to sport (i.e., more than 7 days) was associated with greater symptom load, headaches lasting more than 60 hours, and self-reported fatigue and fogginess (Makdissi et al., 2010). Youth with a history of multiple concussions also are at greater risk for prolonged recovery and PCS (Collins et al., 2002; Eisenberg et al., 2013; Guskiewicz et al., 2003; Kerr et al., 2012; Schatz et al., 2011; Zemper, 2003).
Recent imaging studies have begun to examine the role of depressive symptoms in predicting clinical outcome and brain abnormalities in individuals with mild traumatic brain injury (mTBI) (Chen et al., 2008; Maller et al., 2010; Rao et al., 2012). Functional and structural brain differences were examined between a group of 40 male athletes in their 20s and 30s who had a history of three or more concussions, on average, and a control group of male athletes, none of whom had had a concussion in the past 12 months (Chen et al., 2008). The results showed no performance differences between the groups on a working memory task. However, those individuals with symptoms of both concussion and depression showed the greatest reduction in activity in their prefrontal regions, an area of the brain previously implicated in working memory ability. Furthermore, there was a negative association between activity in this region and depressive symptoms. In contrast, activity in regions previously implicated in emotion and mood disorders (e.g., the cingulate, orbitofrontal cortex and hippocampus) was positively correlated with depressive symptoms. A voxel-based morphometry analysis showed gray matter loss within the cingulate that negatively correlated with depressive symptoms. Finally, depressive symptoms correlated with post-concussive symptoms. These findings suggest that post-concussive symptoms often co-occur with depression and have a shared pathology.
A systematic review of 15 prospective studies of sports concussion and mTBI found that predictors of persistent post-concussive symptoms included being older (adolescent versus child) and having had initial symptoms of headache and loss of consciousness. There also was some evidence to support premorbid conditions as contributing to symptom persistence (e.g., previous concussions, learning difficulties, psychiatric difficulties) (Zemek et al., 2013). The most comprehensive prospective study of sports concussion in youth found that prolonged symptoms were predicted by loss of consciousness, posttraumatic amnesia, and high initial levels of symptomatology (McCrea et al., 2013). A recent emergency department-based prospective study found that previous concussions, high levels of initial symptoms, ages greater than 13 years, and an absence of loss of consciousness predicted symptom persistence (Eisenberg et al., 2013). In that study, there was a dose-response relationship between the number of concussions (two or more versus one or fewer concussions) and how long the symptoms lasted, and a prior concussion that occurred within the previous year was associated with an increased risk for prolonged symptoms (Eisenberg et al., 2013).
SYMPTOMATOLOGY IN PROLONGED RECOVERY AND POST-CONCUSSION SYNDROME
Aside from their duration, the symptoms experienced by individuals with prolonged recovery or PCS are the same as those experienced in the acute phase of the injury (e.g., physical, cognitive, emotional, and sleep), and the same symptom scales and checklists are used to assess and monitor individuals with persistent symptoms (see Chapter 3). There has been one carefully conducted, controlled prospective study of sports concussions in high school and college athletes, with high school students making up about two-thirds of the concussed athletes (n=570) and of the non-concussed athlete controls (n=166) (McCrea et al., 2013). Athletes had been given baseline, preseason testing with respect to symptoms using the Graded Symptom Checklist (GSC), postural stability using the BESS, and cognitive function using the SAC and paper-and-pencil neuropsychological testing. Symptom, cognitive, and postural recovery were assessed at baseline, the time of injury, 2 to 3 hours post injury, several times during the first week following injury, and at 45 or 90 days post injury.3 Neuropsychological testing was done at baseline, 1 to 2 days and 1 week post injury, and at day 45 or 90 post injury. The athletes were divided into prolonged recovery (PR), typical recovery (TR), and non-concussed (NC) groups. Athletes were assigned to the TR group if their total GSC change score from baseline to post-injury day 7 was with within the 95th percentile of change score for the NC over the same period of time (i.e., a change score on the GSC of 5 or less). Athletes were assigned to the PR group if they had a change score on the GSC of 6 or greater between baseline and post-injury day 7.
Based on these criteria, 10 percent of the concussed athletes were assigned to the PR group. There were no baseline differences among the groups with respect to demographics, prior concussion history, or baseline testing in the symptomatic, cognitive, postural, or neuropsychological domains. The PR group had higher levels of symptoms at all assessment points, while the TR group's GSC scores were higher than those of the NC group at day 3, but not beyond that point (see Figure 4-1). Cognitive testing using the SAC showed that the PR group demonstrated differences from the other two groups through day 7, while the TR group had normalized by day 2 (see Figure 4-2). The trajectories of postural stability converged after the 3-hour point and at no point differentiated the PR from the TR groups (see Figure 4-3). The vast majority of neuropsychological measures did not discriminate across recovery groups, and none discriminated between recovery groups and controls at any time-points.
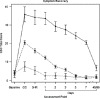
FIGURE 4-1
Symptom recovery curve. NOTES: Figure compares typical recovery (dashed line), prolonged recovery (solid line), and normal control (dotted line) groups. Group × time interaction, p<.001. Higher scores indicate more severe symptoms on the (more...)

FIGURE 4-2
Cognitive recovery curve. NOTES: Figure compares typical recovery (dashed line), prolonged recovery (solid line), and normal control (dotted line) groups. Group × time interaction, p<.001. Lower scores indicate poorer cognitive test performance (more...)

FIGURE 4-3
Postural stability recovery curve. NOTES: Figure compares typical recovery (dashed line), prolonged recovery (solid line), and normal control (dotted line) groups. Group × time interaction, p<.001. Higher scores indicate poorer balance (more...)
Predictors of prolonged recovery included loss of consciousness (odds ratio=4.2, 95% confidence interval, 2.1-8.2); retrograde amnesia (odds ratio=2.2, 95% confidence interval, 1.2-4.1); and posttraumatic amnesia (odds ratio=1.8, 95% confidence interval, 1-3.3). Also, an increase in the GSC of 20 or more from baseline to either the 2- to 3-hour assessment or day 1, conferred a nearly threefold increased risk of a prolonged recovery. At 45 or 90 days following injury, 23 percent of the PR group versus only 5 percent of the TR group had symptoms higher than the control recovery trajectory. In a companion study, the same research group showed that post-concussion migraine was associated with increased symptoms through day 7 but not beyond (Mihalik et al., 2013). Interestingly, although females were more likely to experience posttraumatic migraine, a history of previous migraine was rare in this sample and not contributory to risk.
Another study, which included youth with sports-related concussion as well as other types of mTBI, described the phenomenology and trajectory of different symptom clusters. The study, which looked at PCS symptomatology across four categories (cognitive, somatic, emotional, and behavioral) in children ages 8 to 15 years with mTBI, found that children experiencing prolonged recovery consistently self-reported the presence of symptoms in the somatic and cognitive categories, while parents reported the observation of emotional as well as somatic and cognitive symptoms in their children (Ayr et al., 2009). Although the majority of participants in the study had sports- or recreation-related injuries, 10 percent had Glasgow Coma Scale scores of less than 15; 39 percent experienced a loss of consciousness (for a median time of 1 minute, with a range of 0 to 15 minutes); and abnormalities on neuroimaging, which are indicative of more severe injury than that generally associated with sports-related concussion, did not exclude individuals from the study.
Evaluation of PCS symptoms poses a number of challenges. The experience of and reporting of symptoms are, by nature, subjective; the symptoms associated with PCS are not specific for the condition; and the symptoms are prevalent among the general pediatric population. In some cases, persistent symptoms appear to be an extension of symptoms experienced during the acute phase of the injury that are taking longer than usual to resolve; in other cases, because preexisting conditions or a prior history of problems (e.g., sleep, headache, attentional problems) are predictors of prolonged recovery, a concussion may trigger a recurrence of symptoms or exacerbate an ongoing problem (Leddy et al., 2012). The lack of comprehensive longitudinal studies of sports concussions makes it difficult to more precisely delineate the predictors and symptomatic correlates of recovery and persistence of post-concussive symptoms.
The interrelationship among PCS symptoms poses another challenge for evaluating the condition. Research has shown disturbed sleep to be related to poorer functional outcomes and pain (Milroy et al., 2008; Tham et al., 2012). There is also growing evidence that sleep insufficiency or impairment negatively affects children's learning and behavior (Archbold et al., 2004; Blunden et al., 2001; Gozal and O'Brien, 2004; Sadeh et al., 2003). Pain, particularly chronic headache, is very common in cases of traumatic brain injury (TBI), and it is actually more common in those with mTBI than in those with moderate or severe TBI (Blume et al., 2012; Nampiaparampil, 2008). Concussed high school athletes with headaches 7 days following an injury performed more poorly on neurocognitive testing than did those without headaches (Collins et al., 2003).
Depression has also been associated with concussions up to 14 days post injury in high school and collegiate athletes and has been related to lower neurocognitive performance (Kontos et al., 2012). Researchers using functional imaging and diffusion tensor imaging (DTI) have begun to examine commonalities in the brain circuitry implicated in depression and in mTBI. In a review of 57 DTI studies (40 of TBI and 17 of depression), none of which examined depressive symptoms following TBI, common white matter irregularities were identified in frontotemporal regions of the brain, the internal capsule, and the corpus callosum (Maller et al., 2010). Subsequently a longitudinal study of 14 individuals ages 18 to 65 years with mTBI and no history of previous major depression or previous brain injury was completed. DTI measures were attained within 1 month of injury to examine the predictive validity of DTI for later depression. The results showed an association between Hamilton D ratings of depression over the course of the 1-year follow-up and microstructural abnormalities (lower fractional anisotropy and higher mean diffusivity) in frontotemporal regions of the brain within 1 month of the injury (Rao et al., 2012). Together these findings suggest an overlapping neural circuitry of depression and brain injury with frontotemporal neural circuitry that may increase the risk for depression following brain injury. Depressive symptoms following concussions may be associated with structural and functional brain changes, which may inform the treatment of depression and improve outcomes following brain injury.
Recently much attention has been paid to the long-term relationships between sports-related concussions and suicide, but there has been almost no focus on the risk for depression and suicidal behavior during the immediate (e.g., first week) and subsequent post-concussion recovery period. Only one study was identified that has examined the prevalence of suicidal ideation in athletes undergoing preseason testing (Bailey et al., 2010). In this study, 47 collegiate football players ages 17 to 19 were screened as part of preseason testing with the Concussion Resolution Index—a computerized neurocognitive test (see Chapter 3)—and the Personality Assessment Inventory. Significant correlations were found for subscales of the Personality Assessment Inventory with neurocognitive testing. Specifically, suicidal ideation was correlated with slower simple and complex reaction times. Although 23 percent of the sample had had a previous concussion, the relationship between concussion history and suicidal ideation was not assessed. There are no data to evaluate the short-term risk of suicide or suicidal behavior following sports-related concussions, in part because the extant longitudinal studies have primarily focused on post-concussion symptom inventories, which do not assess suicidal ideation or neurocognitive performance.
A study of the emotional responses of collegiate athletes following concussion or musculoskeletal injury found that the emotional profile of the concussed athletes exhibited significantly elevated fatigue and decreased vigor, while athletes with musculoskeletal injuries exhibited significant increases in anger, which resolved to a pre-injury level within 2 weeks (Hutchison et al., 2009).
CLINICAL MANAGEMENT OF PROLONGED SYMPTOMS AND POST-CONCUSSION SYNDROME
There are few interventions available for addressing prolonged recovery or PCS following concussion. Individuals experiencing prolonged symptoms following concussion may be prescribed pharmacologic or other interventions for the treatment of specific symptoms, but the research on such interventions as well as on more general rehabilitative interventions for prolonged recovery following concussion is limited, especially for youth, as is research on the appropriate time to begin interventions.
Role of Exercise in Management of Persistent Symptoms
There is some evidence that noncontact aerobic exercise may play a role in the rehabilitation of individuals experiencing a prolonged recovery or PCS. As long as the individual avoids additional impacts during the window of vulnerability for repeat injury (see Chapters 2, 3, and 5), it appears that exercise will not negatively affect outcomes in youth recovering from sports-related concussion (McCrea et al., 2009). Although there are no randomized controlled trials (RCTs) studying the effects of exercise in youth with a prolonged recovery following sports-related concussion, studies in animals suggest that the use of exercise may be beneficial in the management of individuals with a prolonged recovery and warrants further research.
Animal studies suggest that exercise increases brain chemicals that promote neuroplasticity and neurogenesis; decreases oxidative stress, which can impair brain cell function and lead to cell death; and reduces neuroinflammation and cognitive dysfunction (Carro et al. 2001; Cotman and Berchtold, 2002; Ding et al., 2004, 2006; Griesbach et al., 2004; Neeper et al., 1995; Piao et al., 2013). In addition, exercise appears to have a beneficial effect on depression in adults (Rimer et al., 2012) and also appears to help prevent depression and anxiety in children and adolescents (Larun et al., 2006), but little is known about the role it might play in the prevention or treatment of post-concussive symptoms. Preliminary studies of the use of exercise to help reduce persistent symptoms following a concussion show there may be a positive effect (Gagnon et al., 2009; Leddy et al., 2010, 2013; Schneider et al., 2013).
The only experimental study of exercise and post-concussive symptoms that has been performed to date examined the impact of exercise on 12 adults with refractory PCS (6 athletes, 6 non-athletes) using a randomized, cross-over design. The exercise condition was associated with a reduction in symptoms of PCS, and that decline was correlated with peak exercise heart rate (Leddy et al., 2010). One additional suggestive study used functional magnetic resonance imaging (fMRI) to observe three groups of four subjects each—adults with PCS who engaged in aerobic exercise treatment, PCS patients treated with "flexibility training," and normal controls—as they performed a cognitive task (Leddy et al., 2013). The PCS exercise group showed fewer symptoms at follow-up than did the PCS "flexibility" group, and the exercise group's fMRI during the cognitive task normalized, whereas the PCS "flexibility" group did not. Although both of these studies are much too small to be definitive, they do suggest that it would be valuable to conduct further studies of the role of exercise in facilitating recovery in pediatric patients with PCS.
Symptom Management
The literature reports various interventions for the management of persistent symptoms of concussion and PCS, although the data to support the efficacy of these interventions in individuals, especially children and adolescents, with sports-related concussions are limited, with virtually no data stemming from RCTs (Giza et al., 2013; Makdissi et al., 2013; Management of Concussion/mTBI Working Group, 2009; Petraglia et al., 2012; Schneider et al., 2013). No RCTs have been conducted to assess the effectiveness of symptom-specific interventions in managing difficulties with sleep, emotional issues (e.g., depression, anxiety), problems with cognition, or headaches in children and adolescents suffering from persistent symptoms of concussion or PCS, and therefore there are insufficient data to show that any intervention enhances recovery or prevents long-term sequelae (Pangilinan et al., 2010). The evidence base underlying post-concussive symptom management in youth includes (1) a quasi-experimental study targeting cognitive symptoms in concussed adolescents with amantadine (Reddy et al., 2013) and open trials in adults with posttraumatic headache following either sports concussion or mTBI (Makdissi et al., 2013; McBeath and Nanda, 1994; Packard, 2000; Weiss et al., 1991); (2) RCTs using cognitive behavior therapy for PCS in adults with mild to moderate TBI, which have been suggestive of a beneficial effect, although all of the studies have had methodological limitations (reviewed in Al Sayegh et al., 2010); and (3) RCTs for symptoms similar to PCS in children and adolescents without a history of concussion, as discussed in the following paragraph.
There are RCTs for the management of such difficulties in nonconcussed pediatric populations, but it is currently not known whether these medications and psychotherapeutic interventions would be as effective in concussed youth. For example, cognitive behavioral therapy (CBT) and melatonin have been demonstrated to be efficacious for the management of insomnia in non-concussed youth (Bendz and Scates, 2010; Clarke and Harvey, 2012; Cortesi et al., 2012; Galland et al., 2012; Gradisar et al., 2011; Hollway and Aman, 2011; Paine and Gradisar, 2011; Quach et al., 2011); cognitive behavioral therapy and selective serotonin reuptake inhibitors have been shown to be efficacious for depression and anxiety (Birmaher et al., 2007; Strawn et al., 2012; Walkup et al., 2008); stimulants have been shown to be helpful for attention and concentration difficulties (Vaughan and Kratovil, 2012); and modest evidence supports the use of certain antiepileptic and calcium channel agents for pediatric migraine (Eccleson et al., 2012; Papetti et al., 2010). It is logical to consider these agents for testing in concussed youth who present with post-concussive symptoms.
Cognitive and psychoeducational interventions early in the course of mTBI, including in pediatric populations, have been shown to decrease symptomatology upon follow-up, but no studies have been done in concussed youth. Ponsford and colleagues (2001) randomized youth with mTBI (but with more severe injuries than most sports concussions, because their Glasgow Coma Scale scores were 13 to 15) to either receiving an educational booklet describing expectable course and coping procedures or treatment as usual. At 3-month follow-up, the youth whose parents received the intervention were less symptomatic than were those in the control group. In adults with mTBI, both unselected and in those deemed to be at "high risk" for PCS, those who received CBT intervention delivered shortly after the injury reported a faster and more complete recovery (Mittenberg et al., 1996; Silverberg et al., 2013). Multifaceted approaches to rehabilitation following a concussion may aid in recovery by addressing the individual's physical, social, and psychological well-being (Bloom et al., 2004; Gagnon et al., 2009; Johnston et al., 2004).
EFFECTS OF PROLONGED RECOVERY ON FAMILY
It is important to note the potential effects of prolonged recovery from concussion on the injured person's family as well. Pediatric TBI, particularly of greater severity, is associated with significant family burden, which in turn can influence child outcomes (Peterson et al., 2013; Rivara et al., 1996). But even the symptoms associated with concussions can disrupt family patterns (Snedaker, 2013). Parents may need to take time off from work to care for a concussed child, who in turn may, if an adolescent, suddenly find him- or herself in need of care at a time when he or she is otherwise becoming increasingly independent. Conversely, siblings may receive disproportionately less time and attention from parents (Snedaker, 2013). Wade and colleagues (2006a,b) reported improvements in behavior in children with moderate to severe TBI and reduction in global distress, depression, and anxiety in parents following family-centered problem solving and online parent interventions respectively. However, the effects on families of prolonged recovery from concussion in children have not been systematically studied and interventions have not been tested in such families.
ACCESS TO CARE FOR INDIVIDUALS WITH PROLONGED RECOVERY
It is important for individuals with a concussion to receive care from providers knowledgeable about concussions, and a number of state concussion laws require that high school athletes with a concussion be cleared to return to play by providers knowledgeable in concussion diagnosis and management. Individuals experiencing a prolonged recovery may have a particular need for knowledgeable and coordinated management of their symptoms. During the past several years, there has been a proliferation of clinics around the United States specializing in the management of youth with sports-related concussion (Pennington, 2013). However, access to this sort of specialized concussion care may be limited for youth who lack sufficient health insurance or financial resources as well as for those living in areas where such specialized care is not readily available. The latter may include individuals living in rural areas of the United States and, potentially, military personnel and their dependents who are stationed in other countries.
Telemedicine (i.e., the use of technology to provide medical consultation over a distance) may be a way to provide access to specialized concussion care for some individuals who would otherwise be unable to access such services. The military and Department of Veterans Affairs (VA) Veterans Health Administration have implemented the use of technologies (e.g., Web-based applications, interactive video teleconferencing, electronic health records) to assist in the identification, acute and long-term management, and rehabilitation of individuals with TBI in military and VA settings (Girard, 2007). Telemedicine has been found to be effective in the examination and treatment of stroke victims by permitting evaluation of the patient by a stroke specialist at a remote location, who then directs treatment of the patient by local providers (Schwamm et al., 2009). The Mayo Clinic in Arizona, which has telemedicine programs for stroke, epilepsy, and neurology (Mayo Clinic, 2011), implemented a "teleconcussion" pilot program in 2011 to help provide concussion expertise to patients and their providers in rural areas of Arizona (Vargas et al., 2012). Research is needed to validate the safety and efficacy of telemedicine for concussion identification and management.
FINDINGS
The committee offers the following findings concerning the treatment and management of prolonged symptoms and PCS:
-
Typically youth athletes recover from a concussion within 2 weeks of the injury, but in 10 to 20 percent of cases the symptoms of concussion persist for a number of weeks, months, or even years.
-
Short-term predictors of prolonged recovery and PCS vary across studies but appear to include older age (adolescent versus child), high initial symptom load, initial presenting symptoms of amnesia and loss of consciousness, and some evidence to support premorbid conditions as contributing to symptom persistence (e.g., previous concussion, learning difficulties, psychiatric difficulties).
-
There is a paucity of prospective studies on the course of recovery for youth from sports concussion, and none of these include pre– high school athletes.
-
There are no randomized clinical trials testing the efficacy of psychosocial or psychopharmacological treatments for children and adolescents with post-concussive symptoms and prolonged recovery.
-
There currently are no data to evaluate the relationship between concussion history and risk of suicide in young athletes because existing post-concussion symptoms inventories do not assess suicidal ideation.
REFERENCES
-
Al Sayegh A, Sandford D, Carson AJ. Psychological approaches to treatment of postconcussion syndrome: A systematic review. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81(10):1128–1134. [PubMed: 20802219]
-
APA (American Psychiatric Association). Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Association; 2013.
-
Archbold KH, Giordani B, Ruzicka DL, Chervin RD. Cognitive executive dysfunction in children with mild sleep-disordered breathing. Biological Research for Nursing. 2004;5(3):168–176. [PubMed: 14737917]
-
Ayr LK, Yeates KO, Taylor HG, Browne M. Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. Journal of the International Neuropsychological Society. 2009;15(1):19–30. [PMC free article: PMC2832119] [PubMed: 19128525]
-
Bailey CM, Samples HL, Broshek DK, Freeman JR, Barth JT. The relationship between psychological distress and baseline sports-related concussion testing. Clinical Journal of Sport Medicine. 2010;20:272–277. [PubMed: 20606512]
-
Bendz LM, Scates AC. Melatonin treatment for insomnia in pediatric patients with attention-deficit/hyperactivity disorder. Annals of Pharmacotherapy. 2010;44(1):185–191. [PubMed: 20028959]
-
Birmaher B, Brent D. AACAP Work Group on Quality Issues. Bernet W, Bukstein O, Walter H, Benson RS, Chrisman A, Farchione T, Greenhill L, Hamilton J, Keable H, Kinlan J, Schoettle U, Stock S, Ptakowski KK, Medicus J. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(11):1503–1526. [PubMed: 18049300]
-
Bloom GA, Horton AS, McCrory P, Johnston KM. Sport psychology and concussion: New impacts to explore. British Journal of Sports Medicine. 2004;38(5):519–521. [PMC free article: PMC1724928] [PubMed: 15388529]
-
Blume HK, Vavilala MS, Jaffe KM, Koepsell TD, Wang J, Temkin N, Durbin D, Dorsch A, Rivara FP. Headache after pediatric traumatic brain injury: A cohort study. Pediatrics. 2012;129(1):e31–e39. [PubMed: 22144708]
-
Blunden S, Lushington K, Kennedy D. Cognitive and behavioural performance in children with sleep-related obstructive breathing disorders. Sleep Medicine Reviews. 2001;5(6):447–461. [PubMed: 12531153]
-
Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like Growth Factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. Journal of Neuroscience. 2001;21(15):5678–5684. [PMC free article: PMC6762673] [PubMed: 11466439]
-
Chen JK, Johnston KM, Petrides M, Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Archives of General Psychiatry. 2008;65(1):81–89. [PubMed: 18180432]
-
Chrisman SP, Rivara FP, Schiff MA, Zhou C, Comstock RD. Risk factors for concussive symptoms 1 week or longer in high school athletes. Brain Injury. 2013;27(1):1–9. [PubMed: 23252433]
-
Clarke G, Harvey AG. The complex role of sleep in adolescent depression. Child and Adolescent Psychiatric Clinics of North America. 2012;21(2):385–400. [PMC free article: PMC3605974] [PubMed: 22537732]
-
Collins MW, Lovell MR, Iverson GL, Cantu RC, Maroon JC, Field M. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51(5):1175–1179. discussion 1180-1181. [PubMed: 12383362]
-
Collins MW, Field M, Lovell MR, Iverson G, Johnston KM, Maroona J, Fu FH. Relationship between postconcussion headache and neuropsychological test performance in high school athletes. American Journal of Sports Medicine. 2003;31(2):168–173. [PubMed: 12642248]
-
Cortesi F, Giannotti F, Sebastiani T, Panunzi S, Valente D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: A randomized placebo-controlled trial. Journal of Sleep Research. 2012;21(6):700–709. [PubMed: 22616853]
-
Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25(6):295–301. [PubMed: 12086747]
-
Covassin T, Elbin R, Nakayama Y. Examination of recovery time from sport-related concussion in high school athletes. Physician and Sportsmedicine. 2010;4(38):1–6.
-
Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. European Journal of Neuroscience. 2006;24(5):1265–1276. [PubMed: 16987214]
-
Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Current Neurovascular Research. 2004;1(5):411–420. [PubMed: 16181089]
-
Eccleston C, Palermo TM, de C. Williams AC, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews. 2012;12:CD003968. [PMC free article: PMC3715398] [PubMed: 23235601]
-
Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013;132(1):8–17. [PubMed: 23753087]
-
Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. Journal of Pediatrics. 2003;142(5):546–553. [PubMed: 12756388]
-
Gagnon I, Galli C, Friedman D, Grilli L, Iverson GL. Active rehabilitation for children who are slow to recover following sport-related concussion. Brain Injury. 2009;23(12):956–964. [PubMed: 19831492]
-
Galland BC, Elder DE, Taylor BJ. Interventions with a sleep outcome for children with cerebral palsy or a post-traumatic brain injury: A systematic review. Sleep Medicine Reviews. 2012;16(6):561–573. [PubMed: 22609124]
-
Girard P. Military and VA telemedicine systems for patients with traumatic brain injury. Journal of Rehabilitation Research & Development. 2007;44(7):1017–1026. [PubMed: 18075958]
-
Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TSD, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R. Evidence-Based Guideline Update: Evaluation and Management of Concussion in Sports. American Academy of Neurology; 2013. (Report of the Guideline Development Subcommittee of the American Academy of Neurology). [PMC free article: PMC3721093] [PubMed: 23508730]
-
Gozal D, O'Brien LM. Snoring and obstructive sleep apnoea in children: Why should we treat. Paediatric Respiratory Reviews. 2004;5(Suppl A):S371–S376. [PubMed: 14980299]
-
Gradisar M, Dohnt H, Gardner G, Paine S, Starkey K, Menne A, Slater A, Wright H, Hudson JL, Weaver E, Trenowden S. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep. 2011;34(12):1671–1680. [PMC free article: PMC3208844] [PubMed: 22131604]
-
Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. [PubMed: 15051152]
-
Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA. 2003;290(19):2549–2555. [PubMed: 14625331]
-
Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Halstead M, Herring SA, Kutcher JS, Pana A, Putukian M, Roberts WO. American Medical Society for Sports Medicine position statement: Concussion in sport. British Journal of Sports Medicine. 2013;47(1):15–26. [PubMed: 23243113]
-
Hollway JA, Aman MG. Pharmacological treatment of sleep disturbance in developmental disabilities: a review of the literature. Research in Developmental Disabilities. 2011;32(3):939–962. [PubMed: 21296553]
-
Hutchison M, Mainwaring LM, Comper P, Richards DW, Bisschop SM. Differential emotional responses of varsity athletes to concussion and musculoskeletal injuries. Clinical Journal of Sport Medicine. 2009;19(1):13–19. [PubMed: 19124978]
-
Iverson G. Predicting slow recovery from sport-related concussion: The new simple-complex distinction. Clinical Journal of Sport Medicine. 2007;17(1):31–37. [PubMed: 17304003]
-
Johnston KM, Bloom GA, Ramsay J, Kissick J, Montgomery D, Foley D, Chen JK, Ptito A. Current concepts in concussion rehabilitation. Current Sports Medicine Reports. 2004;3(6):316–323. [PubMed: 15509473]
-
Kerr ZY, Marshall SW, Harding HP Jr, Guskiewicz KM. Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. American Journal of Sports Medicine. 2012;40(10):2206–2212. [PubMed: 22922518]
-
Kontos AP, Covassin T, Elbin RJ, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Archives of Physical Medicine and Rehabilitation. 2012;93(10):1751–1756. [PubMed: 22503738]
-
Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database of Systematic Reviews. 2006;3:CD004691. [PubMed: 16856055]
-
Lau BC, Collins MW, Lovell MR. Sensitivity and specificity of subacute computerized neurocognitive testing and symptom evaluation in predicting outcomes after sports-related concussion. American Journal of Sports Medicine. 2011a;39(6):1209–1216. [PubMed: 21285444]
-
Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players. American Journal of Sports Medicine. 2011b;39(11):2311–2318. [PubMed: 21712482]
-
Lau BC, Collins MW, Lovell MR. Cutoff scores in neurocognitive testing and symptom clusters that predict protracted recovery from concussions in high school athletes. Neurosurgery. 2012;70(2):371–379. [PubMed: 21841522]
-
Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory postconcussion syndrome. Clinical Journal of Sport Medicine. 2010;20(1):21–27. [PubMed: 20051730]
-
Leddy J, Sandhu H, Sodi V, Baker J, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health: A Multidisciplinary Approach. 2012;4(2):147–154. [PMC free article: PMC3435903] [PubMed: 23016082]
-
Leddy JJ, Cox JL, Baker JG, Wack DS, Pendergast DR, Zivadinov R, Willer B. Exercise treatment for postconcussion syndrome: A pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. Journal of Head Trauma and Rehabilitation. 2013;28(4):241–249. [PubMed: 23249769]
-
Makdissi M, Darby D, Maruff P, Ugoni A, Brukner P, McCrory PR. Natural history of concussion in sport: Markers of severity and implications for management. American Journal of Sports Medicine. 2010;38(3):464–471. [PubMed: 20194953]
-
Makdissi M, Cantu RC, Johnston KM, McCrory P, Meeuwisse WH. The difficult concussion patient: What is the best approach to investigation and management of persistent (>10 days) postconcussive symptoms. British Journal of Sports Medicine. 2013;47(5):308–313. [PubMed: 23479490]
-
Maller JJ, Thomson RH, Lewis PM, Rose SE, Pannek K, Fitzgerald PB. Traumatic brain injury, major depression, and diffusion tensor imaging: Making connections. Brain Research Reviews. 2010;64(1):213–240. [PubMed: 20388528]
-
McBeath JG, Nanda A. Use of dihydroergotamine in patients with postconcussion syndrome. Headache. 1994;34(3):148–151. [PubMed: 8200788]
-
McClincy MP, Lovell MR, Pardini J, Collins MW, Spore MK. Recovery from sports concussion in high school and collegiate athletes. Brain Injury. 2006;20(1):33–39. [PubMed: 16403698]
-
McCrea M, Guskiewicz K, Randolph C, Barr WB, Hammeke TA, Marshall SW, Kelly JP. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery. 2009;65(5):876–882. discussion 882-883. [PubMed: 19834399]
-
McCrea M, Guskiewicz K, Randolph C, Barr WB, Hammeke TA, Marshall SW, Powell MR, Woo Ahn K, Wang Y, Kelly JP. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. Journal of the International Neuropsychological Society. 2013;19(1):22–33. [PubMed: 23058235]
-
McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvoøák J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS, Raftery M, Sills A, Benson BW, Davis GA, Ellenbogen RG, Guskiewicz K, Herring SA, Iverson GL, Jordan BD, Kissick J, McCrea M, McIntosh AS, Maddocks D, Makdissi M, Purcell L, Putukian M, Schneider K, Tator CH, Turner M. Consensus statement on concussion in sport: The 4th International Conference on Concussion in Sport held in Zurich, November 2012. British Journal of Sports Medicine. 2013;47(5):250–258. [PubMed: 23479479]
-
Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Amercian Journal of Sports Medicine. 2013;41(7):1490–1496. [PubMed: 23696213]
-
Milroy G, Dorris L, McMillan TM. Brief report: Sleep disturbances following mild traumatic brain injury in childhood. Journal of Pediatric Psychology. 2008;33(3):242–247. [PubMed: 17967815]
-
Mittenberg W, Tremont G, Zielinski RE, Fichera S, Rayls KR. Cognitivebehavioral prevention of postconcussion syndrome. Archives of Clinical Neuropsychology. 1996;11(2):139–145. [PubMed: 14588914]
-
Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: A systematic review. JAMA. 2008;300(6):711–719. [PubMed: 18698069]
-
Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. [PubMed: 7816089]
-
Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache. 2000;40(9):736–739. [PubMed: 11091292]
-
Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behaviour Research and Therapy. 2011;49(6-7):379–388. [PubMed: 21550589]
-
Pangilinan PH, Giacoletti-Argento A, Shellhaas R, Hurvitz EA, Hornyak JE. Neuropharmacology in pediatric brain injury: A review. PM & R. 2010;2(12):1127–1140. [PubMed: 21145525]
-
Papetti L, Spalice A, Nicita F, Paolino MC, Castaldo R, Iannetti P, Villa MP, Parisi P. Migraine treatment in developmental age: Guidelines update. Journal of Headache and Pain. 2010;11(3):267–276. [PMC free article: PMC3451916] [PubMed: 20349201]
-
Peterson RL, Kirkwood MW, Taylor HG, Stancin T, Brown TM, Wade SL. Adolescents' internalizing problems following traumatic brain injury are related to parents' psychiatric symptoms. Journal of Head Trauma Rehabilitation. 2013;28(5):E1–E12. [PMC free article: PMC3769459] [PubMed: 22935574]
-
Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: A review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520–1533. discussion 1533. [PubMed: 22289786]
-
Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R, Loane DJ, Faden AI. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiology of Disease. 2013;54(June):252–263. [PMC free article: PMC3628975] [PubMed: 23313314]
-
Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Curran C, Ng K. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–1303. [PubMed: 11731651]
-
Quach J, Hiscock H, Ukoumunne OC, Wake M. A brief sleep intervention improves outcomes in the school entry year: A randomized controlled trial. Pediatrics. 2011;128(4):692–701. [PubMed: 21890825]
-
Rao V, Mielke M, Xu X, Smith GS, McCann UD, Bergev A, Doshi V, Pham DL, Yousem D, Mori S. Diffusion tensor imaging atlas-based analyses in major depression after mild traumatic brain injury. Journal of Neuropsychiatry and Clinical Neuroscience. 2012;24(3):309–315. [PMC free article: PMC5646269] [PubMed: 23037644]
-
Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. Journal of Head Trauma Rehabilitation. 2013;28(4):260–265. [PubMed: 22613947]
-
Rimer J, Dwan K, Lawlor DA, Greig CA, McMurdo M, Morley W, Mead GE. Exercise for depression. Cochrane Database of Systematic Reviews. 2012;7:CD004366. [PubMed: 22786489]
-
Rivara JM, Jaffe KM, Polissar NL, Fay GC, Liao S, Martin KM. Predictors of family functioning and change 3 years after traumatic brain injury in children. Archives of Physical Medicine and Rehabilitation. 1996;77(8):754–764. [PubMed: 8702368]
-
Ruff RM. Mild traumatic brain injury and neural recovery: Rethinking the debate. NeuroRehabilitation. 2011;28(3):167–80. [PubMed: 21558623]
-
Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;74(2):444–455. [PubMed: 12705565]
-
Schatz P, Moser RS, Covassin T, Karpf R. Early indicators of enduring symptoms in high school athletes with multiple previous concussions. Neurosurgery. 2011;68(6):1562–1567. discussion 1567. [PubMed: 21258259]
-
Schneider KJ, Iverson GL, Emery CA, McCrory P, Herring SA, Meeuwisse WH. The effects of rest and treatment following sport-related concussion: A systematic review of the literature. British Journal of Sports Medicine. 2013;47(5):304–307. [PubMed: 23479489]
-
Schwamm LH, Holloway RG, Amarenco P, Audebert HJ, Bakas T, Chumbler NR, Handschu R, Jauch EC, Knight WA IV, Levine SR, Mayberg M, Meyer BC, Meyers PM, Skalabrin E, Wechsler LR. on behalf of the American Heart Association Stroke Council and the Interdisciplinary Council on Peripheral Vascular Disease. A review of the evidence for the use of telemedicine within stroke systems of care: A scientific statement from the American Heart Association/American Stroke Association. Stroke. 2009;40(7):2616–2634. [PubMed: 19423852]
-
Silverberg ND, Hallam BJ, Rose A, Underwood H, Whitfield K, Thornton AE, Whittal ML. Cognitive-behavioral prevention of postconcussion syndrome in at-risk patients: A pilot randomized controlled trial. Journal of Head Trauma Rehabilitation. 2013;28(4):313–322. [PubMed: 23640544]
-
Snedaker K. Concerns and Issues Faced by Families of Concussed Youth; Presentation before the committee; Washington, DC. February 25. 2013.
-
Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2012;21(3):527–539. [PubMed: 22800992]
-
Tham SW, Palermo TM, Vavilala MS, Wang J, Jaffe KM, Koepsell TD, Dorsch A, Temkin N, Durbin D, Rivara FP. The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. Journal of Neurotrauma. 2012;29(1):154–61. [PMC free article: PMC3253307] [PubMed: 22029569]
-
Vargas BB, Channer DD, Dodick DW, Demaerschalk BM. Teleconcussion: An innovative approach to screening, diagnosis, and management of mild traumatic brain injury. Telemedicine and e-Health. 2012;18(10):803–806. [PubMed: 23101482]
-
Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America. 2012;21(4):941–955. [PubMed: 23040908]
-
Wade SL, Carey J, Wolfe CR. An online family intervention to reduce parental distress following pediatric brain injury. Journal of Consulting and Clinical Psychology. 2006a;74(3):445–454. [PubMed: 16822102]
-
Wade SL, Michaud L, Brown TM. Putting the pieces together: Preliminary efficacy of a family problem-solving intervention for children with traumatic brain injury. Journal of Head Trauma Rehabilitation. 2006b;21(1):57–67. [PubMed: 16456392]
-
Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine. 2008;359(26):2753–2766. [PMC free article: PMC2702984] [PubMed: 18974308]
-
Weiss HD, Stern BJ, Goldberg J. Post-traumatic migraine: Chronic migraine precipitated by minor head or neck trauma. Headache. 1991;31(7):451–456. [PubMed: 1774160]
-
WHO (World Health Organization). International Statistical Classification of Diseases and Related Health Problems. 2010. [October 3, 2013]. (10th Revision. [Online version.]). http://apps
.who.int/classifications /icd10/browse/2010/en. -
Zemek RL, Farion KJ, Sampson M, McGahern C. Prognosticators of persistent symptoms following pediatric concussion: A systematic review. JAMA Pediatrics. 2013;167(3):259–265. [PubMed: 23303474]
-
Zemper ED. Two-year prospective study of relative risk of a second cerebral concussion. American Journal of Physical Medicine and Rehabilitation. 2003;82(9):653–659. [PubMed: 12960905]
- 1
- 2
-
The ICD-10 uses the term "postconcussional syndrome."
- 3
-
Over the course of the 10-year study, the remote recovery assessment point changed from 90 to 45 days.
Source: https://www.ncbi.nlm.nih.gov/books/NBK185342/
0 Response to "How to Easy Your Sympotoms of Post Concussion Syndrom"
Post a Comment